Access the Latest Cancer Treatments by Participating in a Clinical Trial.
At SCRI, we believe participation in a clinical trial should be the first option in fighting cancer, not the last.®
A cancer diagnosis can be life-altering.
Where you go for care can be the difference between confident care planning and confusion.
Consider these important criteria when choosing your cancer care provider and team:
- Choose a hospital or cancer care center with active clinical trials in cancer.
- Select an oncologist who participates in or leads clinical trials investigating the latest in cancer treatments.
- Confirm the oncologist and care team have access to personalized medicine technology to pinpoint the molecular make-up of your cancer so you can be matched with the most targeted treatment possible.
- Ask your oncologist and their team to explore clinical trials as a first care option, especially if there are tailored treatment options being studied that may be more effective in targeting your particular cancer.
A cancer diagnosis can be life-altering.
Where you go for care can be the difference between confident care planning and confusion.
Consider these important criteria when choosing your cancer care provider and team:
- Choose a hospital or cancer care center with active clinical trials in cancer.
- Select an oncologist who participates in or leads clinical trials investigating the latest in cancer treatments.
- Confirm the oncologist and care team have access to personalized medicine technology to pinpoint the molecular make-up of your cancer so you can be matched with the most targeted treatment possible.
- Ask your oncologist and their team to explore clinical trials as a first care option, especially if there are tailored treatment options being studied that may be more effective in targeting your particular cancer.
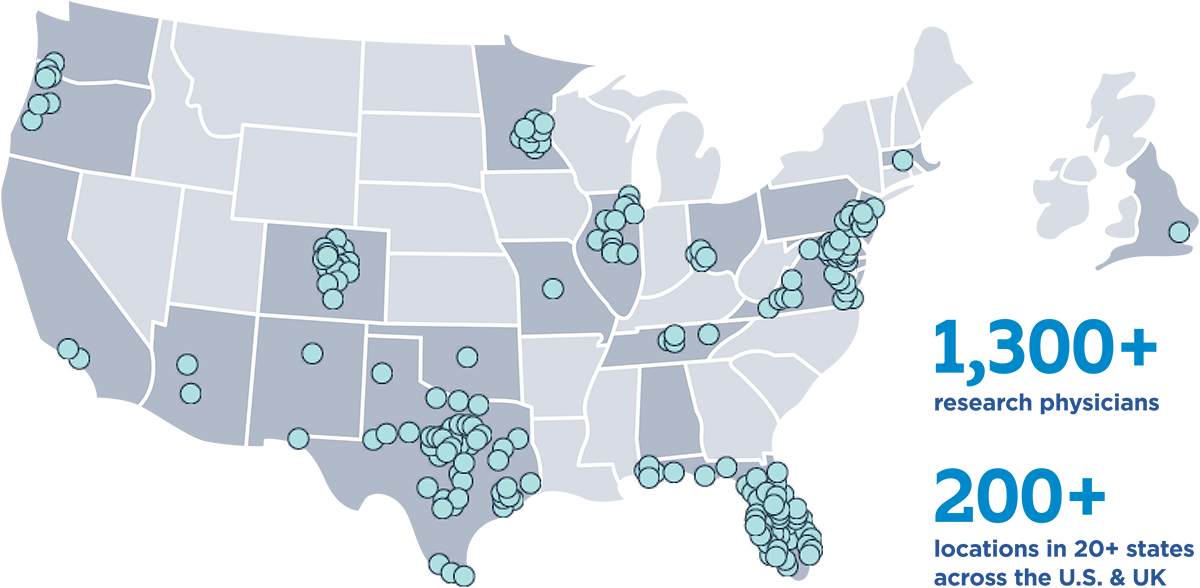
Latest innovations in cancer treatment near you
Where you go for care can be the difference between confident treatment planning and confusion.
Every person facing a cancer diagnosis should have access to the latest innovations in cancer treatment, and at SCRI we are determined to bring cutting-edge therapies available in clinical trials to patients and their families where they live and work.
Explore our network

Why enroll in a cancer clinical trial with SCRI?
Today’s clinical trials are tomorrow’s latest treatment options.
SCRI offers clinical trials with a network of researchers who have trained at the top academic institutions. Each of our research physicians are embedded in community-based oncology practices across the country so that you have the latest treatments and research options closer to where you live.
We also ensure our network of physicians have rapid access to personalized medicine data of your unique cancer through Genospace, a precision medicine technology to help customize your treatment needs.
Read about the benefits of enrollment in a clinical trial and what you can expect in the process.

Ivy
Stage 3b Malignant Melanoma
Ivy, a grandmother from Mississippi, was diagnosed with stage 3b malignant melanoma in the lower leg in 2015. Ivy fought her cancer with numerous surgeries, treatments and radiation but it continued to metastasize to other areas.
Now stage IV, her oncologist referred her to SCRI Oncology Partners in Nashville for a clinical trial after progressing on four prior therapies. Meredith McKean, MD, MPH, director of the melanoma and skin cancer research program at SCRI, first met with her in spring 2023 and set up a plan to target her specific genetic mutations. “Dr. McKean and her staff really listened to my concerns and were good to me, I loved each and every one of them,” Ivy said.
Before she could enroll, she had disease progression in the brain and underwent emergent surgery. She recovered and returned to begin her clinical trial in October 2023. She completed treatment in October 2024 and transitioned follow-up care closer to home to spend more time with her two grandchildren.
“Ivy drove 16 hours roundtrip, every week from Mississippi for treatment and care at SCRI Oncology Partners. Her treatment was not without early side effects, but she tolerated treatment very well and has no active disease,” said Dr. McKean.
Ivy’s recent pet scan in January 2025 was clear and she is enjoying her time at home with family and friends.
“I wish to thank Dr. McKean as well as the sponsors and staff who offered me this clinical trial. I thank God most of all for what he’s done, steering me to SCRI and giving me strength to make weekly trips from Mississippi. My sister calls me a walking miracle but it’s only thru God’s grace I am telling my story today cancer free,” she said.

Jim
Stage 4 Metastatic Prostate Cancer
“Participation in clinical trials should be the first step in fighting cancer, not the last.” – Howard “Skip” Burris, III, MD, President, Sarah Cannon Research Institute (SCRI).
These words ring especially true for SCRI patient, Jim, who in April of 2024 was diagnosed with stage IV metastatic prostate cancer. In July 2024, after enrolling immediately on a clinical trial, scans showed little sign of disease.
Jim, a 20-year healthcare executive from Nashville, Tenn., had kept an eye on a nagging high prostate-specific antigen (PSA) score that recurred within his bloodwork. He and his primary care physician carefully watched the numbers and then within four months, the number tripled. A blood PSA score can indicate higher risk for prostate cancer and, ultimately in Jim’s case, indicted cancer. Jim immediately reached out to Sarah Cannon Research Institute (SCRI) to determine his options.
Jim began care with Benjamin Garmezy, MD, Associate Director, Genitourinary Cancer Research, SCRI and Medical Oncologist, SCRI Oncology Partners who ordered additional testing and scans. These revealed stage IV metastatic prostate cancer with spread to lymph nodes, hips and spine.
“I was absolutely terrified. I didn’t know what to think and it’s still difficult to put into words what it is like to hear the word ‘cancer’,” Jim said. “You never think it is going to happen to you. Then when it does, you are paralyzed.”
Dr. Garmezy suggested an aggressive treatment – first by controlling his testosterone levels, which can fuel prostate cancer, followed by enrollment in a clinical trial aimed to target his type of cancer.
“I am very competitive, type-A personality and a fighter, but you can’t fight something that is internal by yourself. I was reeling from the diagnosis and needed someone to help me make a plan. Dr. Garmezy helped me get off the mat. With that first treatment, I felt like I was doing something to combat the cancer, I could finally fight. With a plan, I felt optimistic.”
Jim enrolled on a clinical trial to intensify his hormonal treatment which includes two daily oral medications and an injection every few months with very little side effects. “I was afraid I would have to trade my vitality to get better. I haven’t - which is amazing. I do have fatigue and a little GI upset but I’ve been able to work and vacation during my battle. I’m just so thankful to maintain quality of life.”
Within three months, Jim’s scans revealed that his cancer is nearly gone. His PSA score went from almost 80 and is now undetectable (a low level is under 4).
“This clinical trial has been a miracle for me and saved my life. I have so much life left to live. I was so fearful this was a death sentence. The people at SCRI have been so amazing to not only me, but to my caretaker as well.”
Jim will remain on the clinical trial as long as it continues to be effective. For Jim, a clinical trial was his first step in fighting cancer and has proven to be successful.

Jimi
Sickle Cell Disease
Jimi, a technology entrepreneur from Atlanta, recently became the world’s first patient with sickle cell disease to summit Mount Kilimanjaro. Jimi took part in SCRI’s CRISPR clinical trial, which is now FDA approved for specific indications, to combat his sickle cell disease which often left him with pain crises and frequent hospitalizations. He received care at Sarah Cannon Research Institute; TriStar Centennial Children’s Hospital under the care of Dr. Haydar Frangoul. Jimi was treated with the CRISPR therapy in fall 2020.
Patients with sickle cell disease are at serious risk of complications and death in low-oxygen environments; doctors advise against entering an elevation of 10,000+ feet. Jimi, now functionally cured, summited Kilimanjaro at 19,341 feet in September 2024! Take a peek at his journey here.
Joseph
Acute Myeloid Leukemia
In the summer of 2023, a physician and dedicated mountain biking coach from Paducah, KY noticed something unsettling: his legs were becoming fatigued quickly, and he was struggling to keep up with his usual pace. During a mountain bike race, he experienced a sharp chest pain. Concerned, he sought medical attention, and his CBC (Complete Blood Count) revealed abnormal levels. He was immediately flown to Nashville, where he was referred to Stephen A. Strickland, Jr. MD, Director of Leukemia Research for Sarah Cannon Research Institute (SCRI).
Joseph was diagnosed with acute myeloid leukemia, a revelation that forced him to make aggressive decisions about his treatment within days. With a history of childhood malignancy and prior chemotherapy, he fell into a rare category. Dr. Strickland and his team discussed the importance of looking for specific biomarkers and exploring newer drugs tailored to his unique case. Soon, he began with a standard chemotherapy regimen.
“The shock of my diagnosis and life changes were significant. I went from being a practicing physician, an active husband and father, and an avid mountain biker to being isolated on a hospital floor in an entirely different state. I was being faced with tough healthcare decisions but as more test results came in, they revealed I was eligible for a new menin inhibitor that could really target my specific kind of cancer,” said Joseph.
His support system was integral to navigating this challenging time. His wife stayed by his side, managing work remotely and helping him make crucial decisions. His professional connections as a vascular neurologist also provided a wealth of advice. But it was Dr. Strickland's compassionate and comprehensive approach that truly made the difference. With each new piece of information, Dr. Strickland updated the treatment options, ensuring that Joseph felt informed and confident in the decisions being made.
Dr. Strickland emphasized the role of clinical trials in providing the best available therapies, underscoring the importance of tailoring treatments to each patient's biology. For Joseph, clinical trials became a lifeline, offering opportunities he never imagined possible. The urgency of having both traditional and research options brought immense comfort.
Once identified as a candidate, he was able to enroll in a clinical trial the very next day, which was an oral medication in combination with traditional chemotherapy. As Joseph progressed through his cancer journey, he returned to mountain biking. His clinical trial was effective and allowed him to return home and to his daily routine.
“Participation in a clinical trial meant not just accessing cutting-edge therapies but getting Joseph back on the mountain trails as both a participant, and a coach to his daughter’s middle school mountain bike team. This is the power of clinical trials,” said Dr. Strickland.
Today, Joseph rides 80 miles a week, is back to work, remains an active member of his church, participates in his mountain biking team, and travels with his family and dogs.
“Reflecting on my journey, I wish I had known from the start that recovery was possible. My journey has profoundly impacted my personal approach to patient care. It has provided me a unique perspective on what it means to be a patient, emphasizing the importance of compassion and genuine communication. I learned firsthand the difference it makes when providers meet patients where they are, a lesson I now carry into my own practice,” said Joseph.
Joseph will remain on the clinical trial and travel to see Dr. Strickland monthly while taking oral medication once daily as part of a planned two-year course of maintenance therapy.

JP
Stage IV Non-Small Cell Lung Cancer
More than a decade ago, JP walked into OHC in Cincinnati, OH with a spirit undeterred by the prognosis of stage IV non-small cell lung cancer. Having exhausted standard treatments, JP sought something more, something that could offer him hope. He enrolled in one of the earliest clinical trials for a revolutionary treatment now known as immunotherapy, offered through a collaboration with Sarah Cannon Research Institute (SCRI).
The clinical trial offered JP a chance, and sometimes, a chance is all a patient needs.
JP responded to the treatment. But he didn't just respond; he thrived. His cancer went into remission, and year after year, his scans remained clear.
“Today, JP is not just a survivor; he’s a beacon of hope. As a volunteer in the clinic, he inspires others with his story, his smile, and his unwavering positivity—showing how clinical research can transform lives,” said David Waterhouse, MD, MPH, Director, Early Phase Clinical Trials at SCRI at OHC.
Every year, JP celebrates the anniversary of his diagnosis with a cake. The first cake read, "55 and still alive." JP, alongside OHC staff, have also celebrated with "57—not in heaven" and "64 and still wanting more." Each year, everyone eagerly awaits his latest creation, confident it will be as uplifting as ever.
Behind the humor and cake lies something profound: a patient who dared to believe in science and a system that made access to it possible. Clinical research is more than studies and protocols; it's a lifeline, a hope for a better tomorrow.
At SCRI at OHC, the mission is clear—bring promising therapies to the patients who need them most, right in their own communities.

Karen
HER 2+ Breast Cancer
Karen, a 66-year-old from Northeast Tennessee, has battled HER 2+ breast cancer since September 2007. After many years of traditional treatment including chemotherapy and radiation, she enrolled on a clinical trial with Dr. Erika Hamilton, Director of Breast Cancer Research, SCRI and a medical oncologist for SCRI Oncology Partners in Nashville, Tenn.
During her time under Dr. Hamilton’s care, Karen enrolled on several clinical trials. Most recently, Dr. Hamilton suggested that Karen try an antibody drug conjugate (ADC) which is a targeted therapy that allows an antibody to bind to proteins on cancer cells, making it easier for the drug to enter a cancer cell and destroy it. ADCs are very targeted, allowing oncologists to use powerful therapies but target it directly to the cancer without harming healthy cells.
“Dr. Hamilton said that antibody drug conjugates are typically easier on the body and I happily obliged!” said Karen.
Karen has been on the antibody drug conjugate for nearly two years now, 42 rounds, and her side effects remain minimal. She continues to work full-time, volunteer in her community and keep up with her busy 16-year-old.
“I feel good! I do have a little hair thinning but for the most part I am able to maintain my life as normal. It has not affected my energy level which I am thankful for so that I can be a healthy, present mother with my daughter as we navigate all of the fun opportunities that high school has to offer.”
Now, in part due to patients like Karen who participate in clinical trials, the antibody drug conjugate is FDA-approved and available commercially to all patients facing cancer.

Kim
Stage 4 Serous Carcinoma Ovarian Cancer
When Kim was diagnosed with stage 4 serous carcinoma ovarian cancer in August 2022, her journey began at St. Mary’s Hospital, marked by the unwavering support of her oncologist, Dr. Krista Isaac. After initial treatments, Kim faced cancer progression, prompting her oncologist to explore clinical trials.
“In any patient with advanced, recurrent cancer I consider if there is a clinical trial option available,” Dr. Isaac said. “This allows a patient to potentially receive a more promising treatment than what I have available as standard of care.”
In January 2024, Kim entered a clinical trial – a pill she took five days on, two days off. Kim trusted her team and stayed on the trial for eight months, experiencing her longest period without progression. When the cancer advanced again, she was offered another clinical trial- an infusion given every three weeks. Her first scan was encouraging, with significant reductions in tumor markers and lesion sizes. And after her fourth infusion, a scan brought more good news: all lesions had decreased in size, some significantly, and no new cancer had appeared.
While the process of joining a trial can be overwhelming with scans, EKGs, bloodwork, and paperwork—Kim credits Lindsay, her clinical research nurse, for making it manageable. “She sets up everything, checks in constantly, and if any billing confusion arises, she handles it,” said Kim.
“Communication is key,” Dr. Isaac added. “My research coordinator contacts our trial patients on a regular basis, and patients have a separate number to call with any questions or issues.”
The support Kim has received from the entire SCRI at Alliance Cancer Specialists team has made a lasting impression. “Everyone from the front desk, to the lab, to the infusion room treats you with kindness. They answer your questions and help lift some of the weight you carry as a cancer patient. When cancer already brings so much stress, that kind of support makes a big difference,” Kim shared.
“I would strongly encourage patients to consider a clinical trial if their physician feels it is a good fit. We’ve developed a robust clinical trial program at Alliance through our relationship with the Sarah Cannon Research Institute (SCRI),” said Dr. Isaac.
Kim's journey highlights the impact of clinical research and the compassionate care at Alliance. Her advice to others considering a trial? “Get informed. Ask every question. Talk with your care team and your loved ones. For me, deciding to do the second trial first meant I still had chemo as a backup. It gave me options, and that matters when you’re living with cancer,” said Kim.
Today, Kim’s gratitude runs deep—for her care team, for the opportunities clinical research has brought, and for the community she’s found through advocacy. She and her family have participated in the Together in Teal walk/run for three years, raising more than $10,000 for the National Ovarian Cancer Coalition.
“We do it not just for me,” Kim said, “but for everyone facing this diagnosis. I’m incredibly thankful to be part of something that could help others down the line too.”
Learn more about Alliance Cancer Specialists

Lisa
Triple-Negative Breast Cancer
“In the world of oncology, every patient story is a testament to resilience, hope, and the relentless pursuit of healing. One particular case of this stands out among my 40 years in oncology and took place in 2006—a young woman pregnant with her first child, received a daunting diagnosis: a left breast mass with lymph node involvement, identified as triple-negative breast cancer. The situation was further complicated by lung metastasis revealed through a chest X-ray,” said Dr. Joyce O’Shaughnessy, Disease Chair, Breast Cancer Research Executive Committee, Sarah Cannon Research Institute (SCRI); Baylor University Medical Center, Texas Oncology, Dallas, TX.
Lisa, then 37, delivered a healthy baby girl —now a young woman in college.
Soon after delivery, Lisa began her cancer journey.
"When I was diagnosed, I was given only a 10% chance of survival. Triple-negative breast cancer is highly aggressive, with five-year survival rates trailing behind other breast cancer types. At the time, my form of cancer represented just 11% of all cases. Confronted with these daunting odds as a new mother, it was an unbelievably challenging period. Yet, here I am, almost 20 years later – a testament to resilience and hope!" shared Lisa.
Lisa enrolled on an investigator-initiated clinical trial at Texas Oncology in 2006. She had a remarkable response, experiencing only a manageable follicular skin rash controlled by antibiotics.
Over time, Lisa displayed a full clinical response – the breast mass, effected lymph nodes and lung metastasis disappeared. Opting for a lumpectomy, she achieved a pathologic complete response, followed by radiation therapy. It was a triumph, and for a time, she enjoyed the freedom from chemotherapy, relying solely on maintenance drugs.
In 2008, surveillance via CT scan revealed a recurrence of lung metastasis. In a collaborative effort with the study sponsor, permission was granted to reinduce her treatment, leading to approximately four cycles. Since then, maintenance therapy has kept her free of disease.
In the years that followed, increased screening uncovered a genetic mutation, prompting a bilateral mastectomy as a preventive measure. Yet, despite these hurdles, she remains in complete response, a testament to the evolving understanding of cancer mutations.
“Reflecting on this journey, I am humbled by the privilege of being part of such a profound success story. It underscores the vital importance of the research we dedicate ourselves to each day. Her story is a beacon of hope, a reminder of the power of medicine and the human spirit,” said Dr. O’Shaughnessy.

Michael
Sickle Cell Disease
Michael, now age 38, was born with sickle cell disease, a blood disorder which causes the body to produce abnormally shaped red blood cells. Sickle cell disease affects approximately 1 out of every 365 Black or African-Americans – causing extremely painful crises that last days and sometimes weeks.
Historically, there have been few treatment options and the pain often disrupts everyday living – making it difficult to go to school, play sports and hold a full-time job.
Luckily, Michael’s mother is a nurse and was able to relieve his pain crises through IV treatments and helped him manage appropriate time limits for physical exertion.
“I loved sports. I opted to be the water boy for my high school football team so that I got to travel with the team although I couldn’t play,” said Michael. “I was first told that I would die by my 18th birthday and if I happened to live past that, I shouldn’t have children because I couldn’t be there for them as they grew up. This was simply not the life I saw for myself.”
Michael struggled with pain crises – oftentimes waiting in the local emergency room for 8-12 hours in hopes of being treated. “Sickle cell disease is especially tricky because you outwardly look fine but, on the inside, your red blood cells wreak havoc on your body. I’ve had fellow sickle cell friends die from a heart attack in the ER while waiting to be seen. I knew there had to be a better way.”
Michael beat the odds and went on to have three beautiful, healthy sons – none of whom have sickle cell disease. He chose to stay close to his mom and his hometown physician in rural Mississippi who helped him advocate for appropriate healthcare interventions. He went to community college and worked three jobs to provide for his family but then one day, everything changed.
“I went to my mom’s medical office on a Saturday to help her take care of a few things. I decided to put out the new health magazines in the waiting room. As I arranged them, there was Victoria Gray on the cover of NPR sharing how she was free from any sickle cell disease symptoms through gene editing. The clinical trial was in Nashville at Sarah Cannon Research Institute (SCRI). I couldn’t believe it- my mom and I had been praying for something just like this!”
Michael immediately decided to search for Victoria on Facebook. Since both Michael and Victoria are from Mississippi, she popped up right away. “Victoria was gracious in sharing with me guidance around the clinical trial option. We had so much in common – we are around the same age; we both have three children and we are both from rural Mississippi. If she could change her life, so could I.”
Michael began the clinical trial under the care of Haydar Frangoul, MD, MS, Medical Director of Pediatric Hematology/Oncology, SCRI and TriStar Centennial Children’s Hospital. “When I found out I was accepted to the trial, my mom and I cried for 30 minutes. I traveled to Nashville every month and had fantastic results. I loved the people at SCRI and the culture of Nashville.”
Now, Michael hasn’t had a pain crisis in two years.
“Now, without these pain crises, I can dream dreams that I couldn’t dream before. Even my mother was able to retire once she knew that I had no symptoms of disease. I am so thankful to Dr. Frangoul and the whole care team. They each went above and beyond, and I’ll be forever grateful.”

Mike
Uveal Melanoma and Colon Cancer
Mike, an avid amateur golfer, former college football player and a 20+ year NCAA football referee, was in excellent health when he awoke on December 12, 2018, and could not see out of his left eye. Mike is a husband, father to twin daughters, Ironman triathlete, successful business owner and, amongst other things, has even built an airplane from scratch in his workshop. Mike never let life slow him down.
“When I couldn’t see well out of my left eye, I thought I had just scratched my eye with something flying around in my workshop. I didn’t think anything of it and headed out to the golf course to walk 18 holes with my clubs on my back,” said Mike. “But on the 9th hole, it became evident that I couldn’t see anything out of that eye. So, I walked off the course and went straight to my optometrist, who happened to be doing my required annual eye exams for NCAA Football.
Mike was immediately referred to an ophthalmologist and then a retina specialist, who determined that Mike had uveal melanoma, or ocular melanoma, which is a kind of cancer that occurs in the eye and can easily spread to other parts of the body. The tumor within his eye had torn his retina, making left eyesight impossible.
Over the next year, Mike had nine eye surgeries, radiation in his eye and spent two months lying face down trying to save his vision. “My retina specialist, Dr. David A. Reichstein at Tennessee Retina, tried his best to save my sight, but his ultimate goal was to save my life. After a year-long battle, he was successful in saving my life, but my vision was permanently lost.”
Because his cancer could come back, Mike was referred to Sarah Cannon Research Institute (SCRI) in February 2020 where he was monitored closely under the care of Meredith McKean, MD, MPH, Director of Melanoma and Skin Cancer Research. When scans revealed that the cancer had reemerged, this time in his liver, Mike was offered an option to go on a clinical trial specifically tailored to his cancer.
“I opted into the trial and began in September 2020, in the height of the COVID-19 pandemic. I made terrific friends with both staff and others facing cancer during this dark time. A few have lost the fight and I wear bracelets every day to remember their battle and remind myself that my fight means something – I wanted to share my story simply to show what is possible through modern medicine, professional healthcare and positive thinking.”
Over the coming months, Mike’s scans began to show a slow in the spread of his cancer and then, eventually, some shrinking of his tumors. He was able to resume daily life and activity but with a few side effects – dizziness, fatigue, joint inflammation and a stronger than usual appetite.
In January 2023, scans showed that the cancerous spots on his liver were nearly gone. He received treatment, through IV, every other Monday for more than two years. He then entered into a battle with stage III colon cancer and finished chemotherapy in summer of 2024. In November 2024, his scans showed no sign of disease. He is no longer on active treatment and comes in every three months for check ups with Dr. McKean at SCRI Oncology Partners.
“Dr. McKean could not believe it and asked to see the results of the scans a second time! This is a medical breakthrough in the fight against cancer. The care I have received at SCRI has saved my life.”
Throughout this fight, Mike continued doing what he loves, playing golf. He recently played in two professional golf events and numerous amateur tournaments, despite being blind in one eye.
“I can say confidently that I am the best partially blind, stage IV cancer fighter, out there! I played the USGA qualifier just three days after my active, seven-hour immunotherapy treatment. In total, I walked nine miles with 12,000 feet of vertical incline and decline throughout the golf course. I committed early on to find things that bring me joy and pursue those things and that is what I have done. Mindset is such a tool in the fight against cancer. I see a long, fruitful life ahead of me and I am so thankful for Sarah Cannon Research Institute and the power of clinical trials.”

Theresa
Stage 4 Metastatic Breast Cancer
Several years ago, Theresa noticed a knot in her right breast, but at the time, she was caring for her mother and delayed visiting her doctor. Following her mother’s passing, Theresa noticed a new lump growing on the crown of her head and scheduled a biopsy with a dermatologist, which revealed a malignant growth. This ultimately led to her being diagnosed with stage 4 metastatic breast cancer.
Following further testing, Theresa’s primary doctor explained that she was a candidate for a clinical trial at Sarah Cannon Research Institute (SCRI) in Nashville, Tenn., where she met Erika Hamilton, MD, Director, Breast Cancer Research; Executive Chair, Breast Cancer Research Committee, SCRI, and began her clinical trial treatment journey under Dr. Hamilton’s supervision using a hormone blocker in combination with a PI3 kinase inhibitor in the first-line setting.
Theresa continues the trial with no visible disease on scans and has maintained a thriving and full life in her retirement. In fact, Theresa recently celebrated 100 months on her phase 1 clinical trial, and the care team at SCRI in Nashville surprised her with a cake to honor this incredible moment.
-

Stage 3b Malignant Melanoma
Ivy
Ivy, a grandmother from Mississippi, was diagnosed with stage 3b malignant melanoma in the lower leg in 2015. Ivy fought her cancer with numerous surgeries, treatments and radiation but it continued to metastasize to other areas.
Now stage IV, her oncologist referred her to SCRI Oncology Partners in Nashville for a clinical trial after progressing on four prior therapies. Meredith McKean, MD, MPH, director of the melanoma and skin cancer research program at SCRI, first met with her in spring 2023 and set up a plan to target her specific genetic mutations. “Dr. McKean and her staff really listened to my concerns and were good to me, I loved each and every one of them,” Ivy said.
Before she could enroll, she had disease progression in the brain and underwent emergent surgery. She recovered and returned to begin her clinical trial in October 2023. She completed treatment in October 2024 and transitioned follow-up care closer to home to spend more time with her two grandchildren.
“Ivy drove 16 hours roundtrip, every week from Mississippi for treatment and care at SCRI Oncology Partners. Her treatment was not without early side effects, but she tolerated treatment very well and has no active disease,” said Dr. McKean.
Ivy’s recent pet scan in January 2025 was clear and she is enjoying her time at home with family and friends.
“I wish to thank Dr. McKean as well as the sponsors and staff who offered me this clinical trial. I thank God most of all for what he’s done, steering me to SCRI and giving me strength to make weekly trips from Mississippi. My sister calls me a walking miracle but it’s only thru God’s grace I am telling my story today cancer free,” she said.
-

Stage 4 Metastatic Prostate Cancer
Jim
“Participation in clinical trials should be the first step in fighting cancer, not the last.” – Howard “Skip” Burris, III, MD, President, Sarah Cannon Research Institute (SCRI).
These words ring especially true for SCRI patient, Jim, who in April of 2024 was diagnosed with stage IV metastatic prostate cancer. In July 2024, after enrolling immediately on a clinical trial, scans showed little sign of disease.
Jim, a 20-year healthcare executive from Nashville, Tenn., had kept an eye on a nagging high prostate-specific antigen (PSA) score that recurred within his bloodwork. He and his primary care physician carefully watched the numbers and then within four months, the number tripled. A blood PSA score can indicate higher risk for prostate cancer and, ultimately in Jim’s case, indicted cancer. Jim immediately reached out to Sarah Cannon Research Institute (SCRI) to determine his options.
Jim began care with Benjamin Garmezy, MD, Associate Director, Genitourinary Cancer Research, SCRI and Medical Oncologist, SCRI Oncology Partners who ordered additional testing and scans. These revealed stage IV metastatic prostate cancer with spread to lymph nodes, hips and spine.
“I was absolutely terrified. I didn’t know what to think and it’s still difficult to put into words what it is like to hear the word ‘cancer’,” Jim said. “You never think it is going to happen to you. Then when it does, you are paralyzed.”
Dr. Garmezy suggested an aggressive treatment – first by controlling his testosterone levels, which can fuel prostate cancer, followed by enrollment in a clinical trial aimed to target his type of cancer.
“I am very competitive, type-A personality and a fighter, but you can’t fight something that is internal by yourself. I was reeling from the diagnosis and needed someone to help me make a plan. Dr. Garmezy helped me get off the mat. With that first treatment, I felt like I was doing something to combat the cancer, I could finally fight. With a plan, I felt optimistic.”
Jim enrolled on a clinical trial to intensify his hormonal treatment which includes two daily oral medications and an injection every few months with very little side effects. “I was afraid I would have to trade my vitality to get better. I haven’t - which is amazing. I do have fatigue and a little GI upset but I’ve been able to work and vacation during my battle. I’m just so thankful to maintain quality of life.”
Within three months, Jim’s scans revealed that his cancer is nearly gone. His PSA score went from almost 80 and is now undetectable (a low level is under 4).
“This clinical trial has been a miracle for me and saved my life. I have so much life left to live. I was so fearful this was a death sentence. The people at SCRI have been so amazing to not only me, but to my caretaker as well.”
Jim will remain on the clinical trial as long as it continues to be effective. For Jim, a clinical trial was his first step in fighting cancer and has proven to be successful.
-

Sickle Cell Disease
Jimi
Jimi, a technology entrepreneur from Atlanta, recently became the world’s first patient with sickle cell disease to summit Mount Kilimanjaro. Jimi took part in SCRI’s CRISPR clinical trial, which is now FDA approved for specific indications, to combat his sickle cell disease which often left him with pain crises and frequent hospitalizations. He received care at Sarah Cannon Research Institute; TriStar Centennial Children’s Hospital under the care of Dr. Haydar Frangoul. Jimi was treated with the CRISPR therapy in fall 2020.
Patients with sickle cell disease are at serious risk of complications and death in low-oxygen environments; doctors advise against entering an elevation of 10,000+ feet. Jimi, now functionally cured, summited Kilimanjaro at 19,341 feet in September 2024! Take a peek at his journey here. -

Acute Myeloid Leukemia
Joseph
In the summer of 2023, a physician and dedicated mountain biking coach from Paducah, KY noticed something unsettling: his legs were becoming fatigued quickly, and he was struggling to keep up with his usual pace. During a mountain bike race, he experienced a sharp chest pain. Concerned, he sought medical attention, and his CBC (Complete Blood Count) revealed abnormal levels. He was immediately flown to Nashville, where he was referred to Stephen A. Strickland, Jr. MD, Director of Leukemia Research for Sarah Cannon Research Institute (SCRI).
Joseph was diagnosed with acute myeloid leukemia, a revelation that forced him to make aggressive decisions about his treatment within days. With a history of childhood malignancy and prior chemotherapy, he fell into a rare category. Dr. Strickland and his team discussed the importance of looking for specific biomarkers and exploring newer drugs tailored to his unique case. Soon, he began with a standard chemotherapy regimen.
“The shock of my diagnosis and life changes were significant. I went from being a practicing physician, an active husband and father, and an avid mountain biker to being isolated on a hospital floor in an entirely different state. I was being faced with tough healthcare decisions but as more test results came in, they revealed I was eligible for a new menin inhibitor that could really target my specific kind of cancer,” said Joseph.
His support system was integral to navigating this challenging time. His wife stayed by his side, managing work remotely and helping him make crucial decisions. His professional connections as a vascular neurologist also provided a wealth of advice. But it was Dr. Strickland's compassionate and comprehensive approach that truly made the difference. With each new piece of information, Dr. Strickland updated the treatment options, ensuring that Joseph felt informed and confident in the decisions being made.
Dr. Strickland emphasized the role of clinical trials in providing the best available therapies, underscoring the importance of tailoring treatments to each patient's biology. For Joseph, clinical trials became a lifeline, offering opportunities he never imagined possible. The urgency of having both traditional and research options brought immense comfort.
Once identified as a candidate, he was able to enroll in a clinical trial the very next day, which was an oral medication in combination with traditional chemotherapy. As Joseph progressed through his cancer journey, he returned to mountain biking. His clinical trial was effective and allowed him to return home and to his daily routine.
“Participation in a clinical trial meant not just accessing cutting-edge therapies but getting Joseph back on the mountain trails as both a participant, and a coach to his daughter’s middle school mountain bike team. This is the power of clinical trials,” said Dr. Strickland.
Today, Joseph rides 80 miles a week, is back to work, remains an active member of his church, participates in his mountain biking team, and travels with his family and dogs.
“Reflecting on my journey, I wish I had known from the start that recovery was possible. My journey has profoundly impacted my personal approach to patient care. It has provided me a unique perspective on what it means to be a patient, emphasizing the importance of compassion and genuine communication. I learned firsthand the difference it makes when providers meet patients where they are, a lesson I now carry into my own practice,” said Joseph.
Joseph will remain on the clinical trial and travel to see Dr. Strickland monthly while taking oral medication once daily as part of a planned two-year course of maintenance therapy.
-

Stage IV Non-Small Cell Lung Cancer
JP
More than a decade ago, JP walked into OHC in Cincinnati, OH with a spirit undeterred by the prognosis of stage IV non-small cell lung cancer. Having exhausted standard treatments, JP sought something more, something that could offer him hope. He enrolled in one of the earliest clinical trials for a revolutionary treatment now known as immunotherapy, offered through a collaboration with Sarah Cannon Research Institute (SCRI).
The clinical trial offered JP a chance, and sometimes, a chance is all a patient needs.
JP responded to the treatment. But he didn't just respond; he thrived. His cancer went into remission, and year after year, his scans remained clear.
“Today, JP is not just a survivor; he’s a beacon of hope. As a volunteer in the clinic, he inspires others with his story, his smile, and his unwavering positivity—showing how clinical research can transform lives,” said David Waterhouse, MD, MPH, Director, Early Phase Clinical Trials at SCRI at OHC.
Every year, JP celebrates the anniversary of his diagnosis with a cake. The first cake read, "55 and still alive." JP, alongside OHC staff, have also celebrated with "57—not in heaven" and "64 and still wanting more." Each year, everyone eagerly awaits his latest creation, confident it will be as uplifting as ever.
Behind the humor and cake lies something profound: a patient who dared to believe in science and a system that made access to it possible. Clinical research is more than studies and protocols; it's a lifeline, a hope for a better tomorrow.
At SCRI at OHC, the mission is clear—bring promising therapies to the patients who need them most, right in their own communities.
-

HER 2+ Breast Cancer
Karen
Karen, a 66-year-old from Northeast Tennessee, has battled HER 2+ breast cancer since September 2007. After many years of traditional treatment including chemotherapy and radiation, she enrolled on a clinical trial with Dr. Erika Hamilton, Director of Breast Cancer Research, SCRI and a medical oncologist for SCRI Oncology Partners in Nashville, Tenn.
During her time under Dr. Hamilton’s care, Karen enrolled on several clinical trials. Most recently, Dr. Hamilton suggested that Karen try an antibody drug conjugate (ADC) which is a targeted therapy that allows an antibody to bind to proteins on cancer cells, making it easier for the drug to enter a cancer cell and destroy it. ADCs are very targeted, allowing oncologists to use powerful therapies but target it directly to the cancer without harming healthy cells.
“Dr. Hamilton said that antibody drug conjugates are typically easier on the body and I happily obliged!” said Karen.
Karen has been on the antibody drug conjugate for nearly two years now, 42 rounds, and her side effects remain minimal. She continues to work full-time, volunteer in her community and keep up with her busy 16-year-old.
“I feel good! I do have a little hair thinning but for the most part I am able to maintain my life as normal. It has not affected my energy level which I am thankful for so that I can be a healthy, present mother with my daughter as we navigate all of the fun opportunities that high school has to offer.”
Now, in part due to patients like Karen who participate in clinical trials, the antibody drug conjugate is FDA-approved and available commercially to all patients facing cancer.
-

Stage 4 Serous Carcinoma Ovarian Cancer
Kim
When Kim was diagnosed with stage 4 serous carcinoma ovarian cancer in August 2022, her journey began at St. Mary’s Hospital, marked by the unwavering support of her oncologist, Dr. Krista Isaac. After initial treatments, Kim faced cancer progression, prompting her oncologist to explore clinical trials.
“In any patient with advanced, recurrent cancer I consider if there is a clinical trial option available,” Dr. Isaac said. “This allows a patient to potentially receive a more promising treatment than what I have available as standard of care.”
In January 2024, Kim entered a clinical trial – a pill she took five days on, two days off. Kim trusted her team and stayed on the trial for eight months, experiencing her longest period without progression. When the cancer advanced again, she was offered another clinical trial- an infusion given every three weeks. Her first scan was encouraging, with significant reductions in tumor markers and lesion sizes. And after her fourth infusion, a scan brought more good news: all lesions had decreased in size, some significantly, and no new cancer had appeared.
While the process of joining a trial can be overwhelming with scans, EKGs, bloodwork, and paperwork—Kim credits Lindsay, her clinical research nurse, for making it manageable. “She sets up everything, checks in constantly, and if any billing confusion arises, she handles it,” said Kim.
“Communication is key,” Dr. Isaac added. “My research coordinator contacts our trial patients on a regular basis, and patients have a separate number to call with any questions or issues.”
The support Kim has received from the entire SCRI at Alliance Cancer Specialists team has made a lasting impression. “Everyone from the front desk, to the lab, to the infusion room treats you with kindness. They answer your questions and help lift some of the weight you carry as a cancer patient. When cancer already brings so much stress, that kind of support makes a big difference,” Kim shared.
“I would strongly encourage patients to consider a clinical trial if their physician feels it is a good fit. We’ve developed a robust clinical trial program at Alliance through our relationship with the Sarah Cannon Research Institute (SCRI),” said Dr. Isaac.
Kim's journey highlights the impact of clinical research and the compassionate care at Alliance. Her advice to others considering a trial? “Get informed. Ask every question. Talk with your care team and your loved ones. For me, deciding to do the second trial first meant I still had chemo as a backup. It gave me options, and that matters when you’re living with cancer,” said Kim.
Today, Kim’s gratitude runs deep—for her care team, for the opportunities clinical research has brought, and for the community she’s found through advocacy. She and her family have participated in the Together in Teal walk/run for three years, raising more than $10,000 for the National Ovarian Cancer Coalition.
“We do it not just for me,” Kim said, “but for everyone facing this diagnosis. I’m incredibly thankful to be part of something that could help others down the line too.”Learn more about Alliance Cancer Specialists
-

Triple-Negative Breast Cancer
Lisa
“In the world of oncology, every patient story is a testament to resilience, hope, and the relentless pursuit of healing. One particular case of this stands out among my 40 years in oncology and took place in 2006—a young woman pregnant with her first child, received a daunting diagnosis: a left breast mass with lymph node involvement, identified as triple-negative breast cancer. The situation was further complicated by lung metastasis revealed through a chest X-ray,” said Dr. Joyce O’Shaughnessy, Disease Chair, Breast Cancer Research Executive Committee, Sarah Cannon Research Institute (SCRI); Baylor University Medical Center, Texas Oncology, Dallas, TX.
Lisa, then 37, delivered a healthy baby girl —now a young woman in college.
Soon after delivery, Lisa began her cancer journey.
"When I was diagnosed, I was given only a 10% chance of survival. Triple-negative breast cancer is highly aggressive, with five-year survival rates trailing behind other breast cancer types. At the time, my form of cancer represented just 11% of all cases. Confronted with these daunting odds as a new mother, it was an unbelievably challenging period. Yet, here I am, almost 20 years later – a testament to resilience and hope!" shared Lisa.
Lisa enrolled on an investigator-initiated clinical trial at Texas Oncology in 2006. She had a remarkable response, experiencing only a manageable follicular skin rash controlled by antibiotics.
Over time, Lisa displayed a full clinical response – the breast mass, effected lymph nodes and lung metastasis disappeared. Opting for a lumpectomy, she achieved a pathologic complete response, followed by radiation therapy. It was a triumph, and for a time, she enjoyed the freedom from chemotherapy, relying solely on maintenance drugs.
In 2008, surveillance via CT scan revealed a recurrence of lung metastasis. In a collaborative effort with the study sponsor, permission was granted to reinduce her treatment, leading to approximately four cycles. Since then, maintenance therapy has kept her free of disease.
In the years that followed, increased screening uncovered a genetic mutation, prompting a bilateral mastectomy as a preventive measure. Yet, despite these hurdles, she remains in complete response, a testament to the evolving understanding of cancer mutations.
“Reflecting on this journey, I am humbled by the privilege of being part of such a profound success story. It underscores the vital importance of the research we dedicate ourselves to each day. Her story is a beacon of hope, a reminder of the power of medicine and the human spirit,” said Dr. O’Shaughnessy.
-

Sickle Cell Disease
Michael
Michael, now age 38, was born with sickle cell disease, a blood disorder which causes the body to produce abnormally shaped red blood cells. Sickle cell disease affects approximately 1 out of every 365 Black or African-Americans – causing extremely painful crises that last days and sometimes weeks.
Historically, there have been few treatment options and the pain often disrupts everyday living – making it difficult to go to school, play sports and hold a full-time job.
Luckily, Michael’s mother is a nurse and was able to relieve his pain crises through IV treatments and helped him manage appropriate time limits for physical exertion.
“I loved sports. I opted to be the water boy for my high school football team so that I got to travel with the team although I couldn’t play,” said Michael. “I was first told that I would die by my 18th birthday and if I happened to live past that, I shouldn’t have children because I couldn’t be there for them as they grew up. This was simply not the life I saw for myself.”
Michael struggled with pain crises – oftentimes waiting in the local emergency room for 8-12 hours in hopes of being treated. “Sickle cell disease is especially tricky because you outwardly look fine but, on the inside, your red blood cells wreak havoc on your body. I’ve had fellow sickle cell friends die from a heart attack in the ER while waiting to be seen. I knew there had to be a better way.”
Michael beat the odds and went on to have three beautiful, healthy sons – none of whom have sickle cell disease. He chose to stay close to his mom and his hometown physician in rural Mississippi who helped him advocate for appropriate healthcare interventions. He went to community college and worked three jobs to provide for his family but then one day, everything changed.
“I went to my mom’s medical office on a Saturday to help her take care of a few things. I decided to put out the new health magazines in the waiting room. As I arranged them, there was Victoria Gray on the cover of NPR sharing how she was free from any sickle cell disease symptoms through gene editing. The clinical trial was in Nashville at Sarah Cannon Research Institute (SCRI). I couldn’t believe it- my mom and I had been praying for something just like this!”
Michael immediately decided to search for Victoria on Facebook. Since both Michael and Victoria are from Mississippi, she popped up right away. “Victoria was gracious in sharing with me guidance around the clinical trial option. We had so much in common – we are around the same age; we both have three children and we are both from rural Mississippi. If she could change her life, so could I.”
Michael began the clinical trial under the care of Haydar Frangoul, MD, MS, Medical Director of Pediatric Hematology/Oncology, SCRI and TriStar Centennial Children’s Hospital. “When I found out I was accepted to the trial, my mom and I cried for 30 minutes. I traveled to Nashville every month and had fantastic results. I loved the people at SCRI and the culture of Nashville.”
Now, Michael hasn’t had a pain crisis in two years.
“Now, without these pain crises, I can dream dreams that I couldn’t dream before. Even my mother was able to retire once she knew that I had no symptoms of disease. I am so thankful to Dr. Frangoul and the whole care team. They each went above and beyond, and I’ll be forever grateful.”
-

Uveal Melanoma and Colon Cancer
Mike
Mike, an avid amateur golfer, former college football player and a 20+ year NCAA football referee, was in excellent health when he awoke on December 12, 2018, and could not see out of his left eye. Mike is a husband, father to twin daughters, Ironman triathlete, successful business owner and, amongst other things, has even built an airplane from scratch in his workshop. Mike never let life slow him down.
“When I couldn’t see well out of my left eye, I thought I had just scratched my eye with something flying around in my workshop. I didn’t think anything of it and headed out to the golf course to walk 18 holes with my clubs on my back,” said Mike. “But on the 9th hole, it became evident that I couldn’t see anything out of that eye. So, I walked off the course and went straight to my optometrist, who happened to be doing my required annual eye exams for NCAA Football.
Mike was immediately referred to an ophthalmologist and then a retina specialist, who determined that Mike had uveal melanoma, or ocular melanoma, which is a kind of cancer that occurs in the eye and can easily spread to other parts of the body. The tumor within his eye had torn his retina, making left eyesight impossible.
Over the next year, Mike had nine eye surgeries, radiation in his eye and spent two months lying face down trying to save his vision. “My retina specialist, Dr. David A. Reichstein at Tennessee Retina, tried his best to save my sight, but his ultimate goal was to save my life. After a year-long battle, he was successful in saving my life, but my vision was permanently lost.”
Because his cancer could come back, Mike was referred to Sarah Cannon Research Institute (SCRI) in February 2020 where he was monitored closely under the care of Meredith McKean, MD, MPH, Director of Melanoma and Skin Cancer Research. When scans revealed that the cancer had reemerged, this time in his liver, Mike was offered an option to go on a clinical trial specifically tailored to his cancer.
“I opted into the trial and began in September 2020, in the height of the COVID-19 pandemic. I made terrific friends with both staff and others facing cancer during this dark time. A few have lost the fight and I wear bracelets every day to remember their battle and remind myself that my fight means something – I wanted to share my story simply to show what is possible through modern medicine, professional healthcare and positive thinking.”
Over the coming months, Mike’s scans began to show a slow in the spread of his cancer and then, eventually, some shrinking of his tumors. He was able to resume daily life and activity but with a few side effects – dizziness, fatigue, joint inflammation and a stronger than usual appetite.
In January 2023, scans showed that the cancerous spots on his liver were nearly gone. He received treatment, through IV, every other Monday for more than two years. He then entered into a battle with stage III colon cancer and finished chemotherapy in summer of 2024. In November 2024, his scans showed no sign of disease. He is no longer on active treatment and comes in every three months for check ups with Dr. McKean at SCRI Oncology Partners.
“Dr. McKean could not believe it and asked to see the results of the scans a second time! This is a medical breakthrough in the fight against cancer. The care I have received at SCRI has saved my life.”
Throughout this fight, Mike continued doing what he loves, playing golf. He recently played in two professional golf events and numerous amateur tournaments, despite being blind in one eye.
“I can say confidently that I am the best partially blind, stage IV cancer fighter, out there! I played the USGA qualifier just three days after my active, seven-hour immunotherapy treatment. In total, I walked nine miles with 12,000 feet of vertical incline and decline throughout the golf course. I committed early on to find things that bring me joy and pursue those things and that is what I have done. Mindset is such a tool in the fight against cancer. I see a long, fruitful life ahead of me and I am so thankful for Sarah Cannon Research Institute and the power of clinical trials.”
-

Stage 4 Metastatic Breast Cancer
Theresa
Several years ago, Theresa noticed a knot in her right breast, but at the time, she was caring for her mother and delayed visiting her doctor. Following her mother’s passing, Theresa noticed a new lump growing on the crown of her head and scheduled a biopsy with a dermatologist, which revealed a malignant growth. This ultimately led to her being diagnosed with stage 4 metastatic breast cancer.
Following further testing, Theresa’s primary doctor explained that she was a candidate for a clinical trial at Sarah Cannon Research Institute (SCRI) in Nashville, Tenn., where she met Erika Hamilton, MD, Director, Breast Cancer Research; Executive Chair, Breast Cancer Research Committee, SCRI, and began her clinical trial treatment journey under Dr. Hamilton’s supervision using a hormone blocker in combination with a PI3 kinase inhibitor in the first-line setting.
Theresa continues the trial with no visible disease on scans and has maintained a thriving and full life in her retirement. In fact, Theresa recently celebrated 100 months on her phase 1 clinical trial, and the care team at SCRI in Nashville surprised her with a cake to honor this incredible moment.
