Accelero
Our accelerated operations model, coordinated through our CRO, is revolutionizing how clinical trials are delivered.

Reimagining what’s possible in oncology clinical trial delivery
Conducting clinical trials is complex, involving research institutions, trial investigators and biopharma partners working together to advance innovative oncology therapies for patients. Sites face difficulties in identifying and enrolling patients, managing numerous external contract research organizations (CROs), conducting multiple trials simultaneously, entering a high volume of time-sensitive data manually, and using multiple disparate electronic data capture (EDC) systems.
Accelero™ is a scaled, seamless operations model that is designed to accelerate drug development by streamlining and optimizing every phase of the clinical trial lifecycle. Accelero is the only model that capitalizes on an integrated CRO-site network to simplify the entire clinical trial process to maximize results.
A game-changer and industry disruptor, Accelero speeds up the clinical trial process, making it more efficient to conduct studies. From faster site activations to seamless data integration, Accelero removes barriers for the teams at clinical sites who make trials possible, while also addressing the challenges and easing the burdens faced by biopharma partners. By streamlining clinical trial delivery, Accelero helps bring cutting-edge cancer therapies to patients faster and more efficiently.
For more information about Accelero,
Watch Here
Conducting clinical trials is challenging –
Accelero is the solution
The traditional clinical trial model is fragmented and struggles to keep pace with the demands of modern research, making today’s clinical trials increasingly difficult to conduct. For instance, there is greater pressure than ever to enroll a representative number of U.S. participants in pivotal oncology clinical trials that support FDA approval.
For pharma and biotech companies, complexities in the traditional model can translate into:
- Slow start-up and lengthy trial timelines
- Delayed patient recruitment/difficulty meeting accrual targets
- Difficulties meeting patient diversity targets
- Issues receiving timely and high-quality data
- Limited connectivity with community oncology sites
The challenges biopharma partners face in regulatory compliance, patient recruitment and data management can impede progress in cancer research, ultimately delaying the entry of innovative therapies to the market.
Learn how we’re partnering to overcome barriers and transform clinical trial delivery.
How Accelero differs from the traditional CRO model
through all phases of the clinical trial lifecycle.
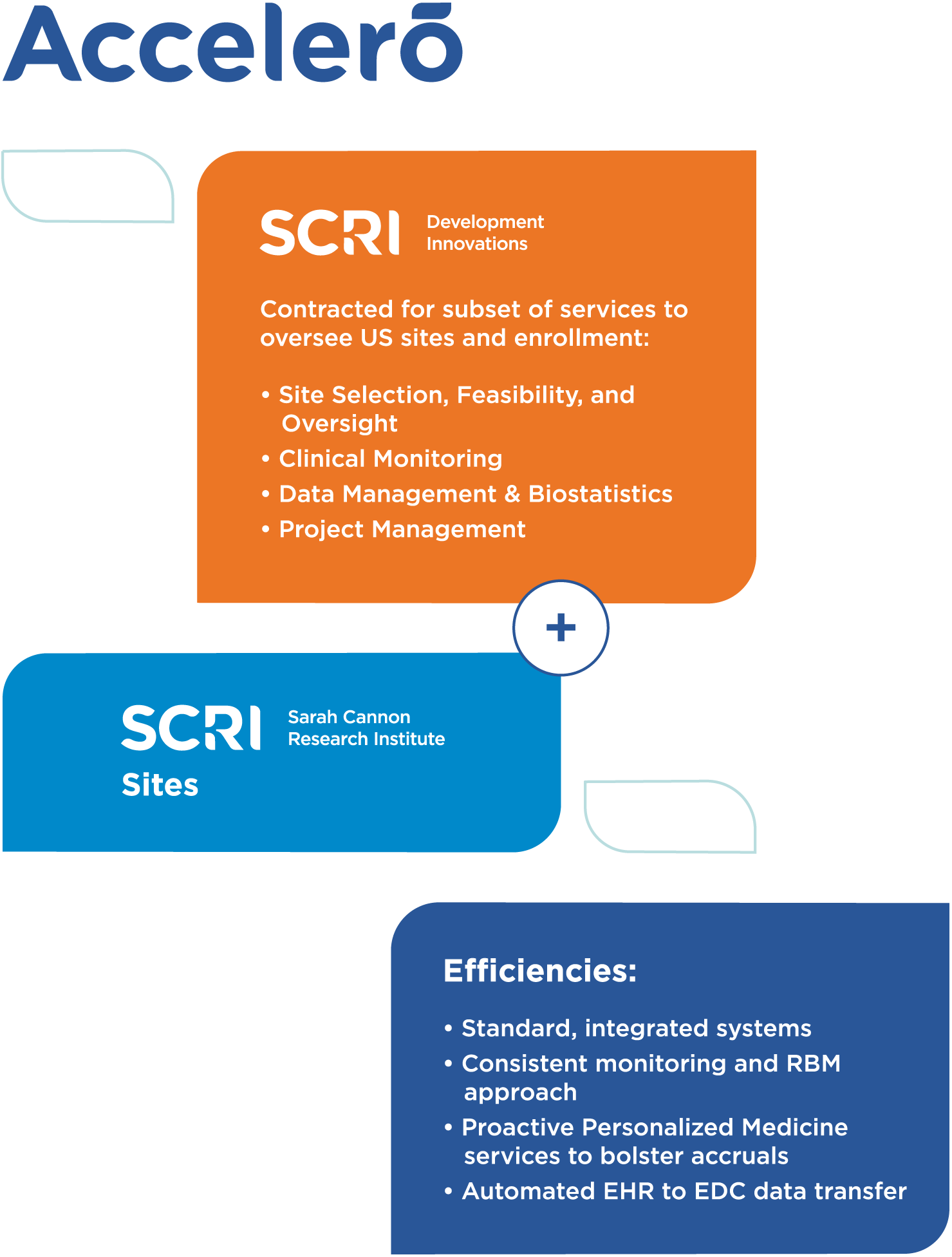
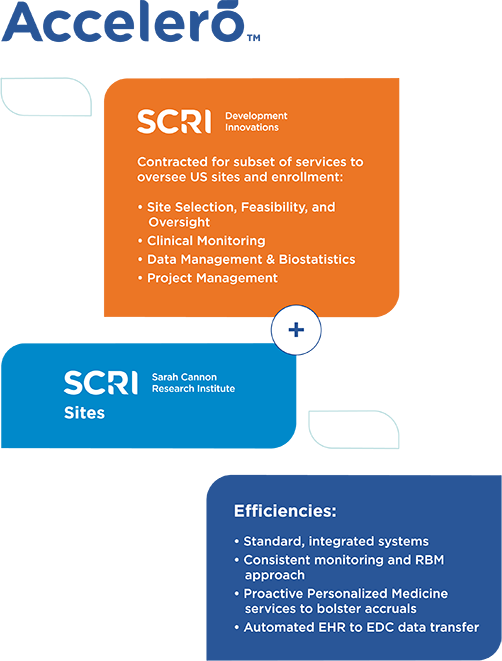
Accelerating and transforming clinical trial delivery with Accelero
SCRI created Accelero to revolutionize how clinical trials are delivered and accelerate trial timelines. Accelero helps sites execute trials more efficiently and inclusively, while reducing administrative burdens and time on the back end. By dismantling barriers, Accelero plays an integral role in helping biopharma partners bring life-changing cancer treatments to patients faster.
Accelero creates clinical trial site efficiencies and standardized ways of working, which translates into the following benefits for biopharma partners:
Accelero creates clinical trial site efficiencies and standardized ways of working, which translates into the following benefits for biopharma partners:
Faster Trial Activation
Accelero accelerates site activation by designing better clinical trials built for the realities of community-based research. Through early site engagement, optimized feasibility processes, and deep connectivity between our CRO and site management organization (SMO), we can reduce start-up timelines and deliver studies more efficiently, which results in time and cost savings for biopharma partners.
Faster Patient Recruitment and Enrollment
Accelero reduces the site administrative burden so researchers can focus on enrolling patients on trials. Accelero also harnesses the power of advanced data analytics to swiftly identify eligible patients. This data-driven approach enables sites to enroll diverse sets of participants and initiate clinical trials more rapidly, helping biopharma partners meet recruitment targets.
Faster Access to Data
Accelero removes the challenges of manual data entry by automating clinical trial data collection. By seamlessly integrating data from the electronic health record (EHR) to the electronic data capture (EDC), Accelero ensures secure, efficient and compliant handling of data, as well as improved data quality. With quicker access to data, biopharma partners can make earlier data-driven decisions about the regulatory strategies for their investigational products.
How Accelero differs from the traditional CRO model
through all phases of the clinical trial lifecycle.

Providing tailored service offerings to meet specific biopharma partner needs
Accelero services are delivered by our oncology-focused CRO, SCRI Development Innovations. Because we understand that each biopharma partner has individual needs, our CRO offers both full and partial scope CRO services. Learn more about our full-service CRO model.
